Mollier Diagram Superheated Steam
Saturated steam 100 C 1 bar after heated to a superheated state at 110 C gaining 30 kJ kg 1 of energy if it is used as a drying medium. Mollier diagram is the h-s diagram of pure substance.

Analysis Of The Mollier Diagram To Simplify The Calculations Of Thermodynamic Magnitudes Steemit
Understanding steam tables and navigating through them is an essential skill associated with thermodynamics.
Mollier diagram superheated steam. Mollier Diagram For Water Steam. Figure 3 shows the schematic diagram of basic principle of a closed system superheated drying system. Download and print Mollier Diagram for Water - Steam.
Superheated Steam Spirax Sarco. Constant specific volume lines 3. The bold line spanning from left to right in the lower half of Mollier diagram is the saturation line.
Fixing the value of any two properties defines the value of all the others. Its quality or dryness fraction x of steam is defined as the ratio of the mass of the vapour to the total mass of both vapour and liquid. The excess energy 2 7417 - 2 7067 35 kJkg and this is used to raise the temperature of the steam from the saturation temperature of 120 C to 136 C.
Pertama kita cari kondisi untuk uap jenuh. Properties of Saturated Steam - Imperial Units - Steam table with sensible latent and total heat and specific volume at different gauge pressures and temperatures. A mollier diagram has the following lines.
The properties in Steam. As the pressure increases the saturation temperature increases and so the slope of the isobar also increases. Diagram Mollier Diagram Mollier merupakan sebagian kecil data dari tabel uap yang diplot ke dalam grafik entalpi h entropi s.
Theso-calledheatdiagramorpdiagraminwhichthe stateof a mass of steam is represented by a point on aplanewith absolute temperature and entropy asrectangularcoor-. The Mollier diagram is a is plot of enthalpy h versus entropy s as shown in Fig. A newly developed gs-state diagram for water and steam is presented in which the specific entropy s is the abscissa while pressure p and Celsius temperature t are used as parameters.
Superheated steam at 700 psia and 680F is expanded at constant entropyto 140 psia. Water Steam Critical And Triple Point. P-v diagram showing the dryness fraction of.
The saturation line labeled as x 1 represents the set of points on Mollier diagram where the steam is 100 vapor. Mollier or Enthalpy-Entropy h-s diagram. The lower portion contains the values of wet steam whereas the upper portion contains the values of superheated steam.
In this state of steam all the liquid particles turn to gas. Dengan menggunakan diagram Mollier kita dapat mengetahui kondisi yang diperlukan untuk daerah jenuh dan superheat. The Mollier diagram is useful when analyzing the performance of adiabatic steady-flow processes such as.
Table A6E Superheated water Table A7E Compressed liquid water Table A8E Saturated icewater vapor Figure A9E T-s diagram for water Figure A10EMollier diagram for water Table A11ESaturated refrigerant-134a Temperature table Table A12ESaturated refrigerant-134aPressure table Table A13ESuperheated refrigerant-134a. The degree of superheat can be determined either by using superheated steam tables or by using a Mollier chart. As discussed above superheated steam occurs when temperature of the steam is raised above the vaporization point.
Thus fixing the values of Enthalpy and Entropy is sufficient to define Temperature Pressure and Internal Energy of the steam. Exhaust end steam moisture content will increase causing blade nozzle and diaphragm erosion. The steam table and mollier diagram of superheated steam are given as shown in Table The lower portion contains the values of wet steam whereas the upper portion contains the values of superheated steam.
Appendix E Steam Tables Fundamentals Of Chemical Engineering. See also Water - Enthalpy H and Entropy S for figures and tabulated values at varying temperatures. Untuk steam superheated fraksi kekeringan steam harus lebih besar dari 1.
Properties Of Saturated Steam Si Units. The diagram is divided into two portions by a somewhat horizontal line termed as saturation curve. KJkg MJkg kcalkg BTUlb.
Hence the constant pressure lines diverge from one another. And that for dry saturated steam x 1. You will get introduced to Mollier diagram and learn how to utilize it in the assessment of enthalpy and entropy changes as temperature and pressure are changed.
This diagram has a series of constant temperature lines constant pressure lines constant quality lines and constant volume lines. The term Mollier diagram named after Richard Mollier 1863-1935 refers to any diagram that features Enthalpy on one. What is thechange in enthalpy.
Use of Mollier Chart. See also 2018 Camry Xle Interior Colors. Dryness fraction lines 2.
Superheated steam is steam heated to a temperature higher than its boiling point corresponding to the operating pressure. Ammonia I Nh Sub 3 Thermodynamic Properties. At point A x 0 At point B x 1 Between point A and B 0 x 10 Note that for a saturated liquid x 0.
Mollier Diagram or steam tables allow determination of the energy available in a pound of steam for a specific pressure and temperature. Specific Heat of Superheated Steam. Untuk kondisi steam jenuh fraksi kekeringan steam harus antara 0 dan 1.
The enthalpy of superheated steam is much higher than saturated steam for a given pressure and temperature. Superheated steam Masing-masing tabel uap tersebut memuat besaran-besaran berikut. What is superheated steam.
Table 523 describes the Mollier Diagram and the parameters involved. Locate point 1 at theintersection of the 700 psia and the 680F. Constant pressure lines 4.
Specific Enthalpy of Superheated Steam. Diagrams of the Properties of Water and Steam. The critical isobar is a.
Specific Volume of Superheated Steam. It can be shown on Mollier diagram. The Mollier diagram is used only when quality is greater than 50 and for superheated.
Use the Mollier Chart. All points above the saturation line are in the superheated steam realm. Mollier Diagram for Water-Steam - Enthalpy-entropy diagram for water and steam.
It is also known as the h-s diagram. The following two examples illustrate the useof the Mollier diagram and the steam tables. Entropy of Superheated Steam - Entropy of steam superheated to temperatures above saturation point.
Steam Tables with Mollier Diagrams SI. The Mollier diagram is useful when analyzing the performance of adiabatic steady-flow processes such as flow in nozzles diffusers turbines and compressors.
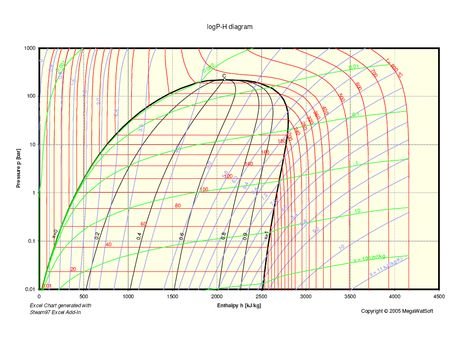
Analysis Of The Mollier Diagram To Simplify The Calculations Of Thermodynamic Magnitudes Steemit

Mollier Diagram For Calculations On Properties Of Steam
Using Mollier S Chart What Is The Final Pressure And Temperature Of Steam When It Is Isentropically Expanded From Enthalpy Of 3500 Kj Kg And 30 Bar Pressure To Enthalps Of 2900 Kj Kg Quora

Steam Boilers Types Click Visit And Get More Ideas Steam Boiler Boiler Steam
De 5 Lesson 23 Use Of Steam Tables Mollier Chart Numerical Problems

Mollier Chart How To Read Youtube

Turbine Expansion Process On The H S Mollier Diagram Drawn Using Download Scientific Diagram

Diagram Mollier For Boiler And Turbine How To Calculation Noakmech
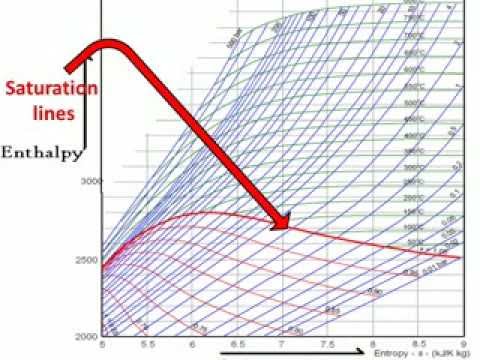
How To Read Mollier Diagram Easy Explain Youtube
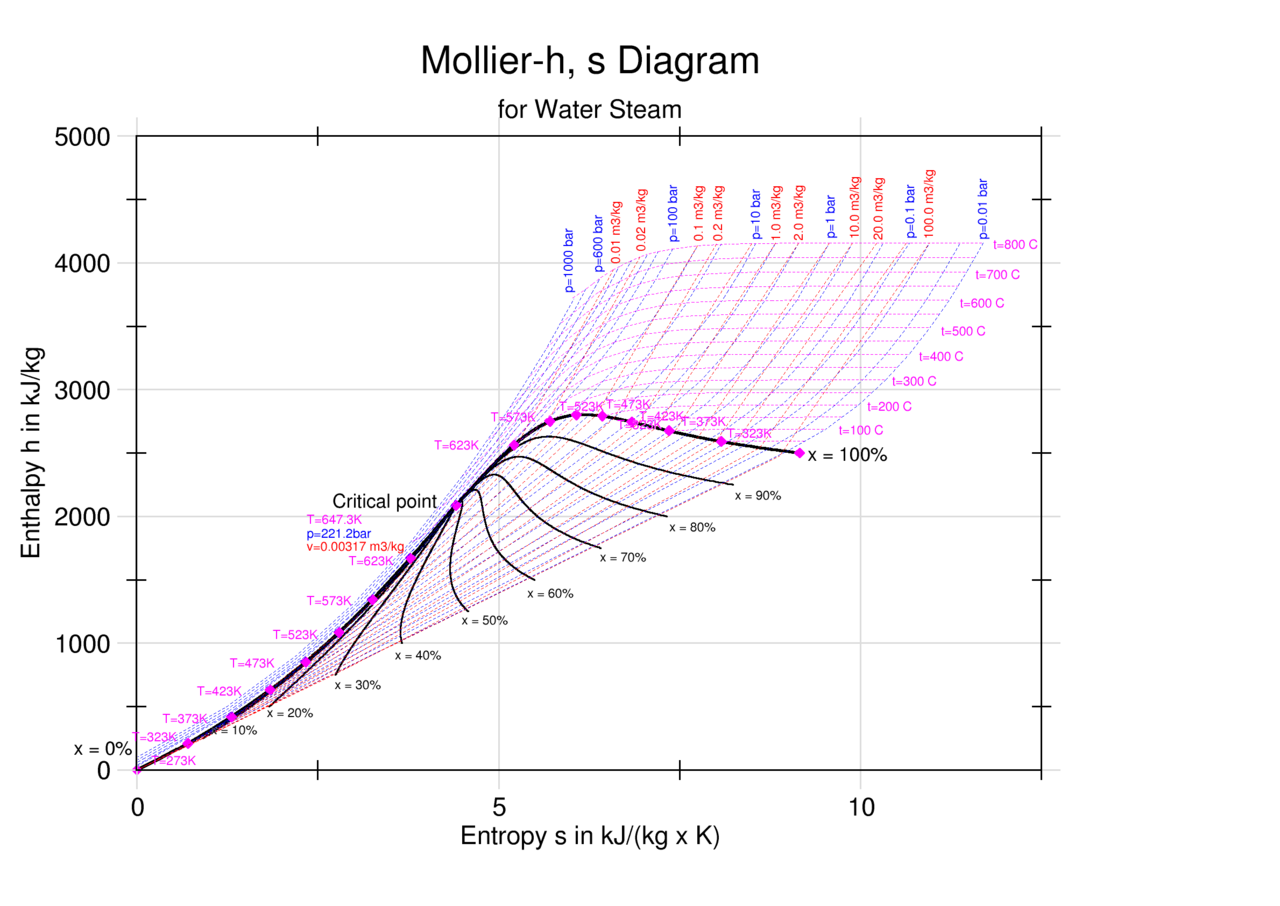
Enthalpy Entropy H S Or Mollier Diagram Engineers Edge
Diagram Mollier Adalah Plot Entalpi Pdf

Steam Expansion Process In Mollier H S Diagram For Three Observed Download Scientific Diagram
Superheated Steam Spirax Sarco
 Reviewed by admin
on
November 11, 2021
Rating:
Reviewed by admin
on
November 11, 2021
Rating:



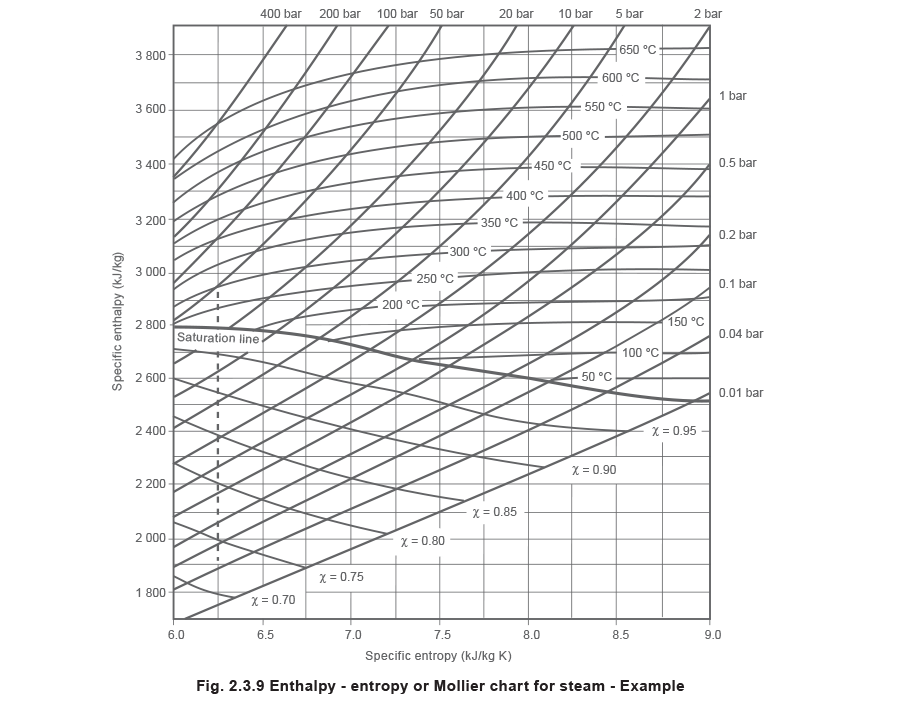
Post a Comment