D Diagram Orbital
Orbital diagram of Oxygen O 9. Each sub-orbital can have a.
Konfigurasi Elektron Dan Diagram Orbital
D orbitals In addition to s and p orbitals there are two other sets of orbitals which become available for electrons to inhabit at higher energy levels.

D diagram orbital. Metal d-orbitals is of great fundamental importance in organometallic and inorganic chemistry. Out of these five d orbitals shapes of the first four d-orbitals are similar to each other which is different from the d z2 orbital whereas the energy of all five d. Orbital diagram of Carbon C 7.
Konfigurasi elektron adalah distribusi dari elektron suatu atom pada orbital-orbital molekulnya. At the third level there is a set of five d orbitals with complicated shapes and names as well as the 3s and 3p orbitals 3px 3py 3pz. Ml0 1 2 Explore other atomic orbitals.
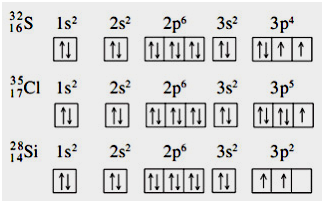
Orbital diagram of Boron B 6. Atom Oksigen memiliki nomor atom 8 dengan konfigurasi elektron 1s 2 2s 2 2p 4Kita akan menentukan diagram orbital dari konfigurasi elektron terakhir yaitu 2p 4. Diagram orbital bisa menggambarkan mengapa ada zat yang warnanya ungu hijau atau bahkan tidak berwarna walaupun ia merupakan logam transisi.
Orbital diagram of Beryllium Be 5. Orbital diagram of Hydrogen H 2. Orbital diagram of Helium He 3.
It allows one to predict and explain the structure reactivity spectroscopic transitions and magnetic properties of transition metal complexes. These orbitals are designated as d xy d yz d xz d x2y 2 and d z2. Orbital diagram of Nitrogen N 8.
Misalnya pada logam transisi yang tidak berwarna Zn bila kita gambarkan diagram orbitalnya akan terlihat perbedaan diagram orbital antara logam itu dengan logam transisi berwarna lain. Orbital diagrams use the same basic format but instead of numbers for the electrons they use and arrows as well. That means that two of the d orbitals will now have a higher energy than the other three - which is exactly what the diagram we have been using shows.
Diagram orbital molekul atau diagram OM adalah suatu alat deskriptif kualitatif yang menjelaskan ikatan kimia dalam molekul dalam hal teori orbital molekul secara umum dan kombinasi linear orbital atom secara khusus. Pada penyusunan diagram orbital sebuah elektron disimbolkan dengan anak panah menghadap ke atas yang melambangkan elektron dengan spin ½ atau menghadap ke bawah. Hence we can say that there are five d-orbitals.
As weve established link to last lesson bonding and antibonding interactions are the key to molecular orbital theory. The Aufbau principle the Pau. There are a total of five d orbitals and each orbital can hold two electrons.
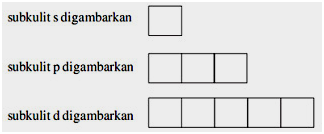
Orbital diagrams are a visual way to show where the electrons are located within an atom. Therefore d-orbital splitting diagrams are an essential part of chemical education so that students develop. CatatanSubkulit s terdiri dari 1 orbital subkulit p 3 orbital subkulit d 5 orbital dan subkulit f 7 orbital.
The d Orbitals Click Here for Sample Questions The magnetic orbital quantum number for d orbitals is denoted as -2 -1 0 1 2. The first number is the principal quantum number n and the letter represents the value of l angular momentum quantum number. Konfigurasi Elektron dan Diagram Orbital.
Diagram Orbital Pengertian dan Contohnya. A general d-orbital splitting diagram for square planar D 4h transition metal complexes can be derived from the general octahedral O h splitting diagram in which the d z 2 and the d x 2 y 2 orbitals are degenerate and higher in energy than the degenerate set of d. In the nickelNi ground-state electron configuration the eight electrons of the 3d orbital are located in the d xy d yz d zx d x 2-y 2 and d z 2 sub-orbitals.
In fact the idea of molecular orbitals the distribution of electrons across a molecule arises from how electrons distribute themselves energetically. The lower energy Square planar and other complex geometries can also be described by CFT. This video explains s p d and f orbitals sublevels and their shapes.
The d-orbital has five sub-orbitals. N represents the energy leve. S-orbitals 2p-orbitals 3p-orbitals 3d-orbitals 4f-orbitals.
Seperti yang telah kita ketahui sebelumnya bahwa struktur atom terdiri dari elektron yang mengelilingi inti atom yang berisi neutron dan proton. Orbital diagram of Fluorine F 10. But two of the d orbitals have lobes pointing along those axes - the 3d x 2 - y 2 and 3d z 2 orbitals.
The d orbitals are given the designations d xy d yz d xz d x 2-y 2 and d z 2. Crystal Field Theory CFT is a model that describes the breaking of degeneracies of electron In a tetrahedral crystal field splitting the d-orbitals again split into two groups with an energy difference of Δtet. Thus there exist five d orbitals.
Molecular Orbital Diagram. Orbital Diagram of All Elements Diagrams. Square planar d z2x2-y d xy d yzxz d z2 d x2-yxy d yz d xz d z2 d x2-y2 d xy d yz d xz d z2 d x2-y d xy d yz d xz z x y.
D-orbital splitting diagrams Use crystal field theory to generate splitting diagrams of the d-orbitals for metal complexes with the following coordination patterns. 1 s 2 p 3 d and 4 f for the orbital and the superscript number tells you how many electrons are in that orbital. The shapes of the first four d orbitals are similar to one another while being different from the dz2 orbital.
Berikut contoh penyusunan diagram orbital. Square pyramidal d z2x2-y d xy d yzxz 5. The transition metal series is defined by the progressive filling of the 3d orbitalsThese five orbitals have the following ml values.
Metal d orbitals in an O h crystal field If a transition metal ion is placed in a spherical field equivalent to the charges on six ligands the energies of all five d orbitals would rise together degenerately as a result of the repulsions between the negative charges on the ligands and the negative charges of the electrons in the metalorbitals. Orbital diagrams must follow 3 rules. Orbital diagram of Lithium Li 4.
Square Planar D Orbital Splitting Diagram. It discusses the 4 quantum numbers n l ml and ms. Orbital adalah bagian dari subkulit atom sebagai daerah yang paling mungkin ditempati elektron.
Those will feel more repulsion than the other three which have lobes in between the axes. Sedangkan diagram orbital adalah deskripsi gambaran dari elektron yang menempati orbital-orbital atom. Orbital adalah wilayah atau daerah dalam ruang di sekitar inti atom yang memiliki kemungkinan tertinggi untuk bisa menemukan elektron.
The sub-orbitals are d xy d yz d zx d x 2-y 2 and d z 2. Prinsip dasar dari teori-teori ini adalah bahwa ketika atom terikat membentuk molekul sejumlah orbital atom bergabung membentuk jumlah orbital. Dalam penyusunan diagram orbital sebuah elektron disimbolkan dengan anak panah menghadap ke atas atau menghadap ke bawah.

Diagram Orbital Dan Konfigurasi Mekanika Kuantum Amruid

Konfigurasi Elektron Pengertian Macam Aturan Rumus Contoh

Diagram Orbital Dan Konfigurasi Mekanika Kuantum Amruid

Qualitative Molecular Orbital Diagram For Moc The 10 Orbital Is Download Scientific Diagram

Diagram Of Orbital Energies With Illustrations Of S P And D Orbital Types Teaching Chemistry Chemistry Notes Chemistry

Suatu Unsur Mempunyai Lambang 199q Diagram Orbital Dari Konfigurasi Unsur Tersebut Adalah Mas Dayat

Menentukan Diagram Orbital Youtube

Prinsip Aufbau Diagram Orbital Konvigurasi Elektron Dalam Kimia
Konfigurasi Elektron Dan Diagram Orbital

Five D Orbitals In A Cubic Crystal Field Which Split Into Two E G Download Scientific Diagram
Bilangan Kuantum Bentuk Orbital Atom Contoh Soal

Qualitative Molecular Orbital Diagram For Moc The 10 Orbital Is Download Scientific Diagram

1 Write Orbital Diagrams For Each Of These Ions A V5 B Cr3 C Ni2 D Fe3 2 Determine If The Ion Is Diamagnetic Or Paramagnetic A V5 B Cr3 C Ni2

Menentukan Diagram Orbital Youtube
 Reviewed by admin
on
December 25, 2021
Rating:
Reviewed by admin
on
December 25, 2021
Rating:

Post a Comment